| Back to |
STUDIES ON ELECTROSTATIC PRECIPITATION
AT TEMPERATURES AROUND ACID DEWPOINT
| Back to |
STUDIES ON ELECTROSTATIC PRECIPITATION
AT TEMPERATURES AROUND ACID DEWPOINT
Takuya YAMAMOTO, Mitsuhiro MIENO,
Kenji SHIBATA, Kazuyoshi TAKAHASHI
Research & Development Center
Sumitomo Heavy Industries, Ltd.
63-30, Yuuhigaoka, Hiratsuka, Kanagawa-Ken, Japan
ABSTRACT
Electrostatic Precipitators (ESP) for coal-fired boilers are generally operated at a temperature above acid dewpoint. Recently, an alternative technique has been developed from the view point of high efficiency, in which the ESP is operated below acid dewpoint temperature. Because of the formation of sulfuric acid mist, the collecting performance of the ESP is improved. This formation is mainly due to condensation of sulfuric acid vapor, created by the reaction between H
2O and sulfur trioxide (SO3) vapor, in the stack gases.In this paper we describe an experimental study of the collecting performance of an ESP at temperatures around acid dewpoint, and the behavior of condensed SO
3. The results may be summarized as follows:(1) The collecting performance of the ESP is improved by the effect of condensed SO
3. (2) Condensed SO3 exists as sulfuric acid mist on the surface of dust particles. (3) At a temperature below acid dewpoint, it is possible to remove SO3 and dust particles from the stack gas with high efficiency.INTRODUCTION
The combustion gases from a coal-fired boiler consist of inert gas, reactive gases such as sulfur dioxide(SO
2), fly ash particles, and condensable vapors (sulfuric acid and water vapor). The ESP is the most widely used system for solid particulate removal from these gases. In the smoke plume, the opacity may increase where hot stack gas is mixed with the cooler atmosphere. This increased opacity is due to aerosol formation resulting from the condensation of vapors, such as sulfuric acid, present in the stack gases. Sulfuric acid is formed by the reaction between S03 and water vapor. The plume opacity of stack gas has been calculated by various authors1),2). The ESP is generally operated above acid dewpoint. In cold-side ESPs the injection of S03 into the gas stream has been attempted in order to improve the collecting performance3). On the other hand, the ESP can remove sulfuric acid mist and dust particles simultaneously from stack gas at temperatures below the acid dewpoint. In addition the plume opacity can be decreased by the removal of sulfuric acid mist. Fig. 1 shows the SO3 condensation on the surface of dust particles in the stack gas. It is considered that gaseous SO3, SO3 mist and dust particles bearing condensed SO3 mist influence the collecting performance of the ESP. We therefore investigated the behavior of SO3 at temperatures around that of acid dewpoint.
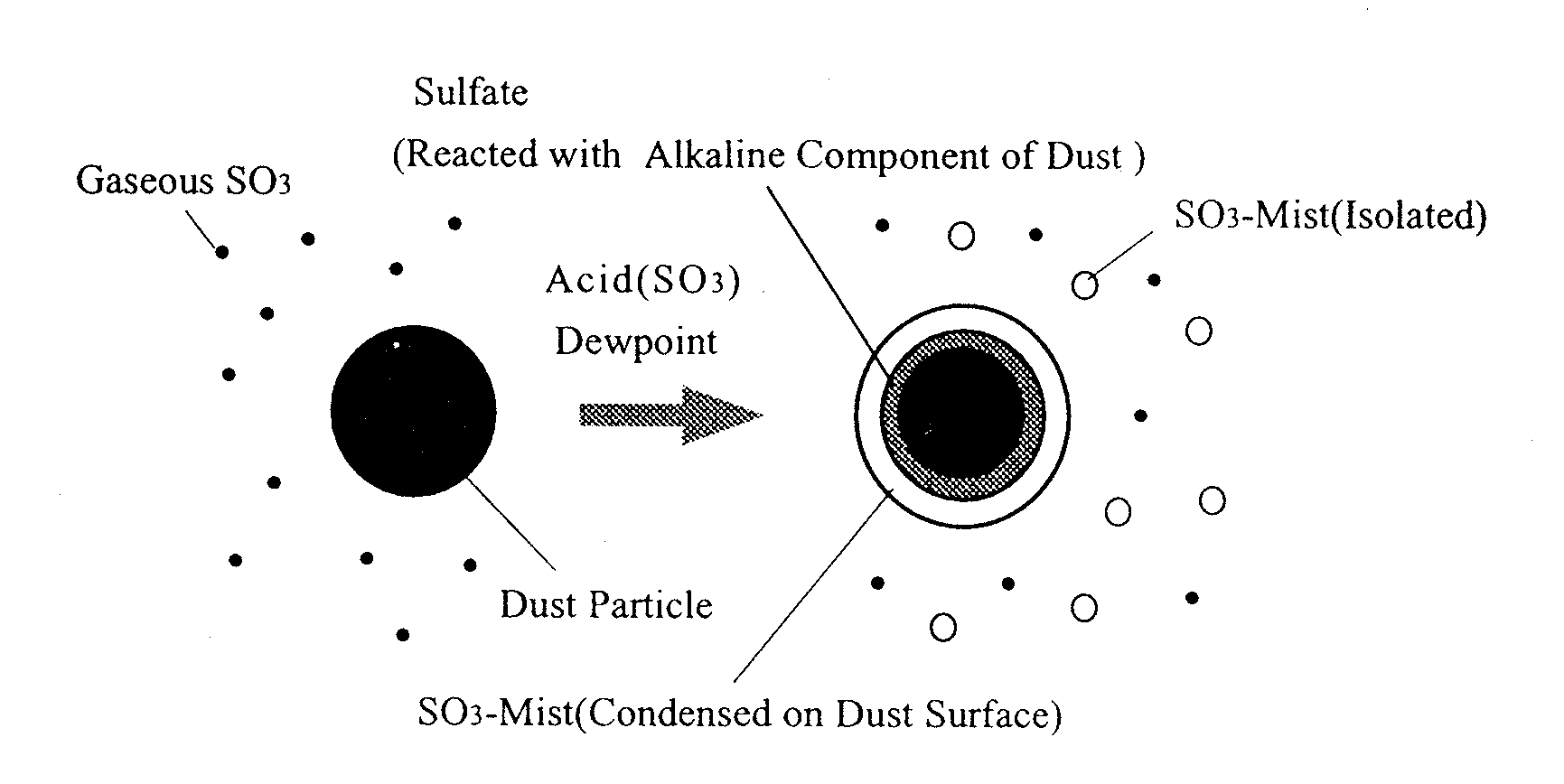
Fig.1 Various Forms of SO
3 below Acid DewpointMETHOD
Electrostatic Precipitator
A schematic of a typical ESP (Max flow rate : 1500m
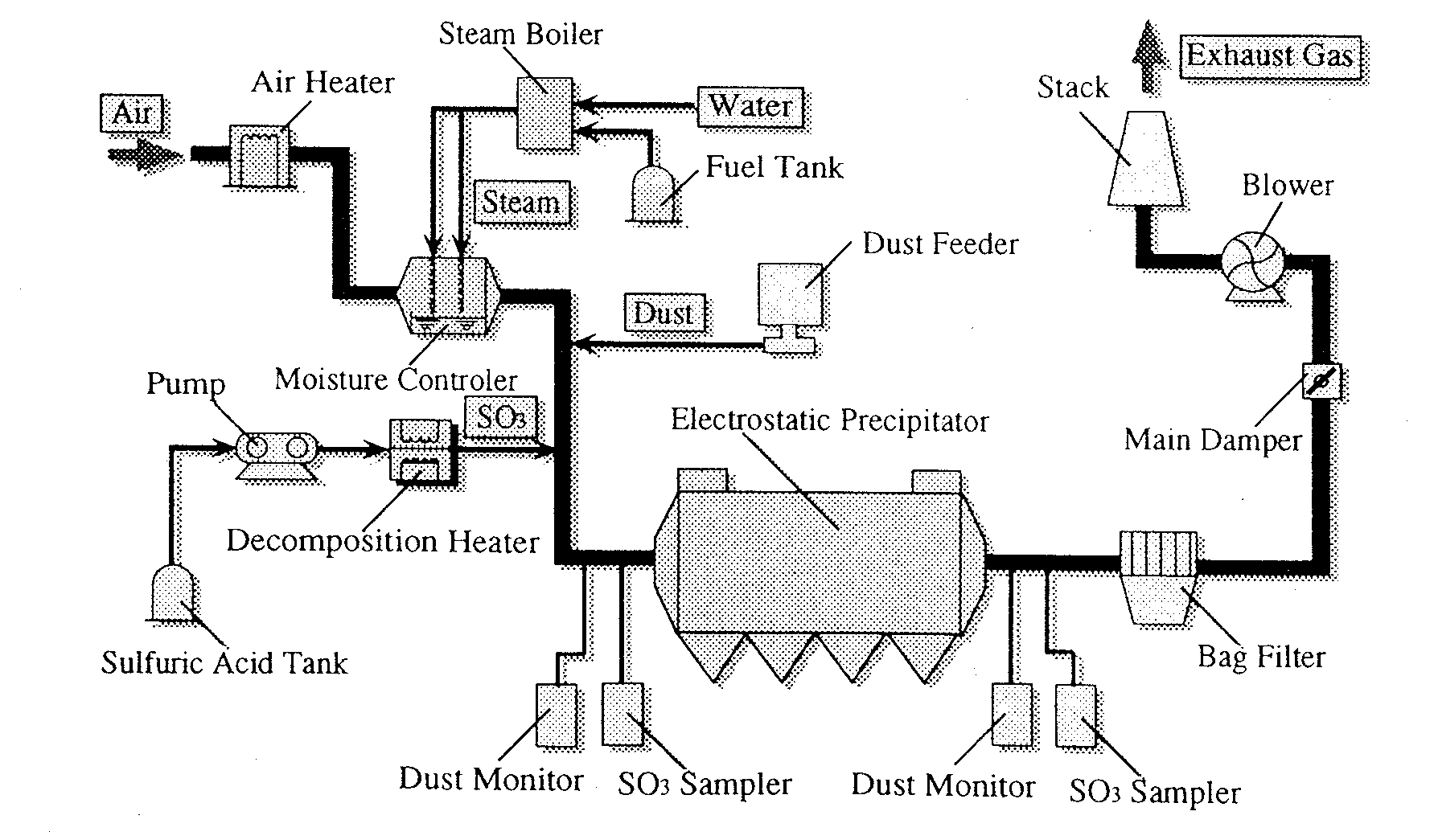
Fig. 2 Schematic of ESP(Max 1500m3N/h)
Table.1 Experimental Conditions
_________________________________________
Gas velocity 1.3m/s
H2O Concentration 8 vol %
SO3 Concentration 0, 1 0 ppm
Average Current Density
0 . 2 mA/m2
at the Plate Electrode
Average Electric Field
1 9 0
~ 4 6 0 kV/m
Force
_____________________________________________
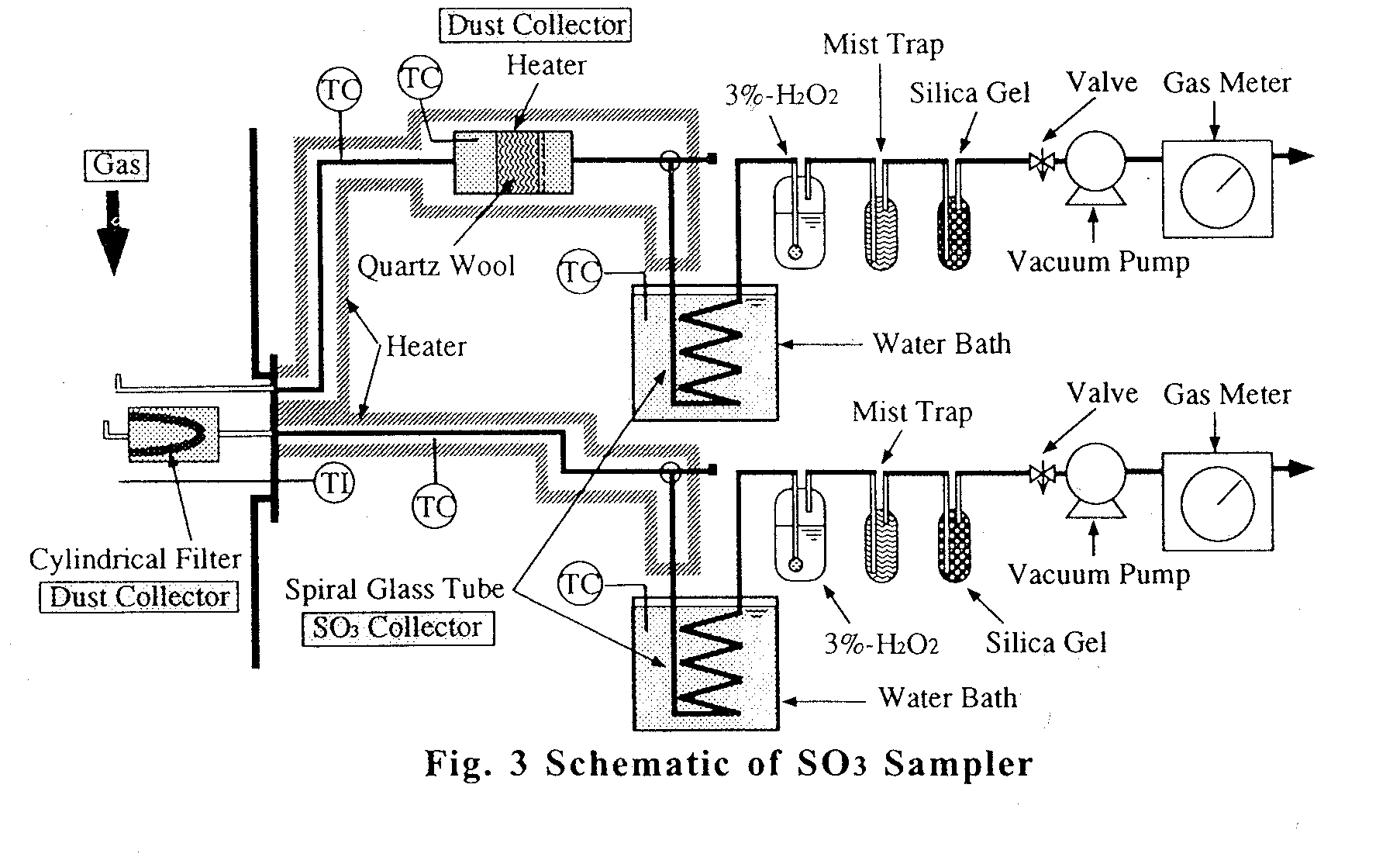
Experimental SO
3 Sampling ApparatusAn SO
3 sampler is shown in Fig.3. Using this sampler, the amount of gaseous SO3(1), SO3-mist(2) and formed sulfate(3) in the stack gas can be individually determine . Dust particles in the stack gas are separated by a dust collector heated to 250OC (packed with quartz wool). SO3-Mist(2) in the stack gas is vaporized by this collector. Vaporized SO3-mist(2) and gaseous SO3(1) are condensed in a spiral glass tube(SO3 Collector) at 70OC. The amount of formed sulfate(3) is determined by analysis of the separated dust particles in the heated dust collector. The total amounts SO3-mist(2) and gaseous SO3
RESULTS AND OBSERVATIONS
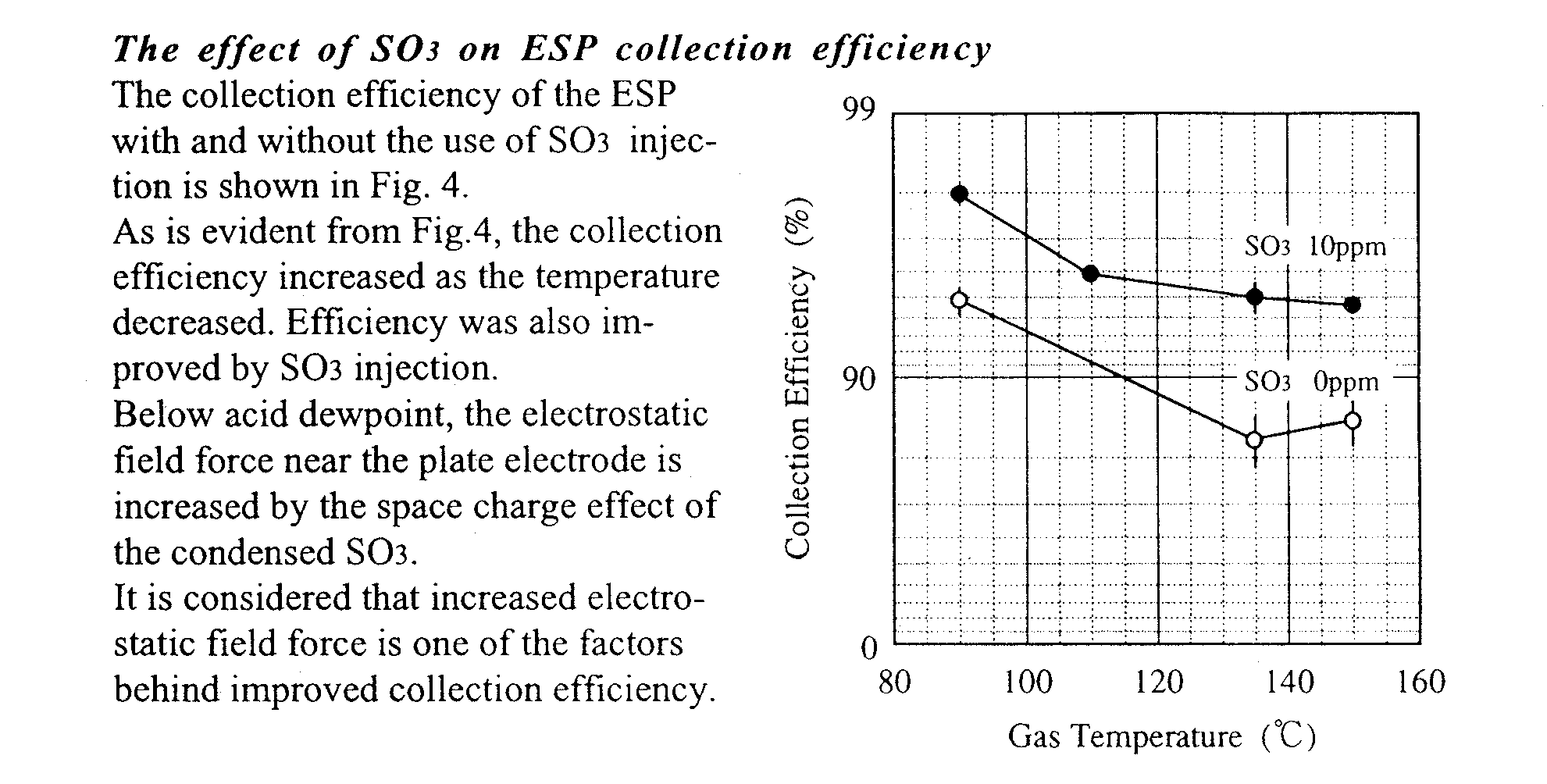
Condensation of SO3 onto dust particles
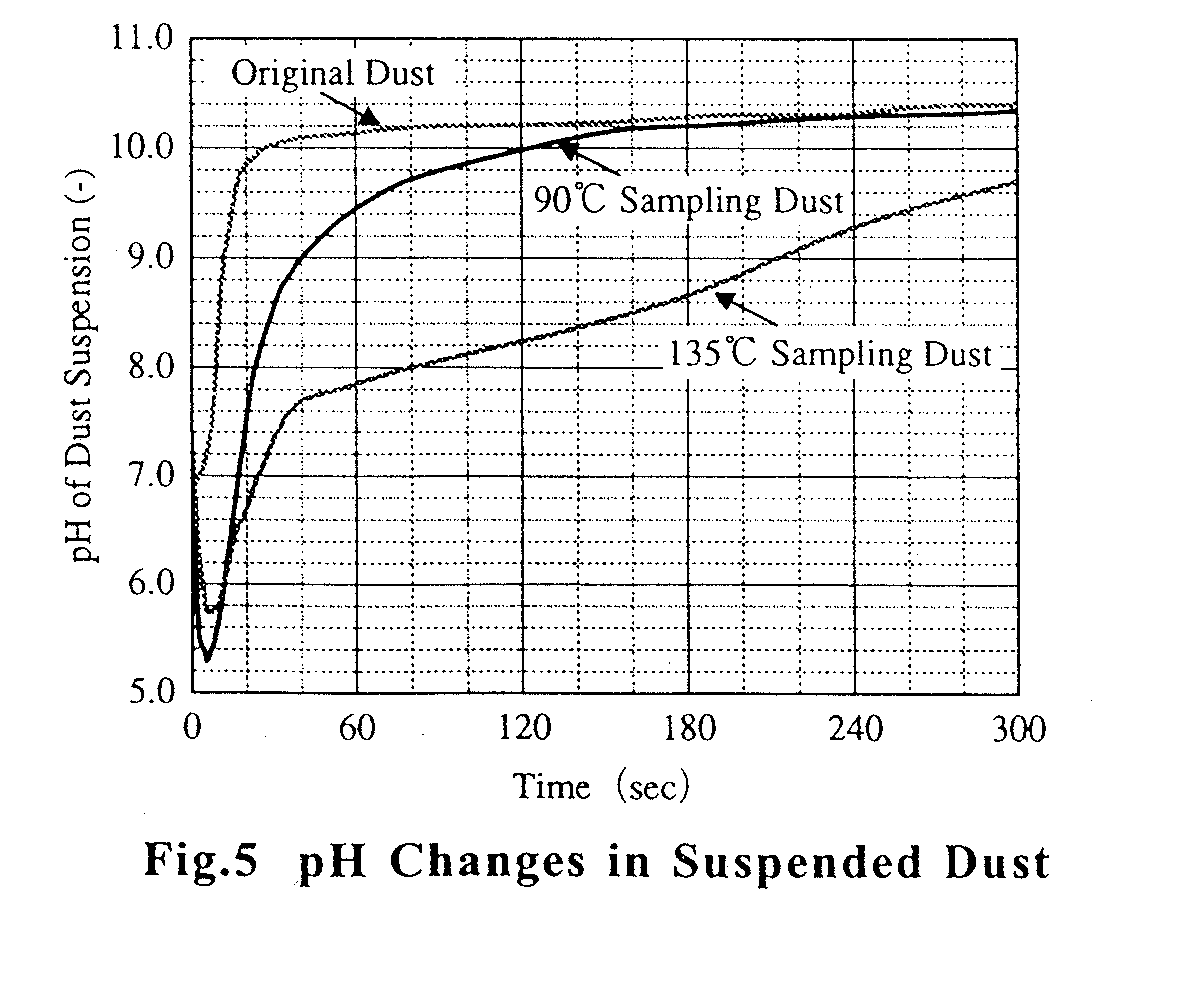
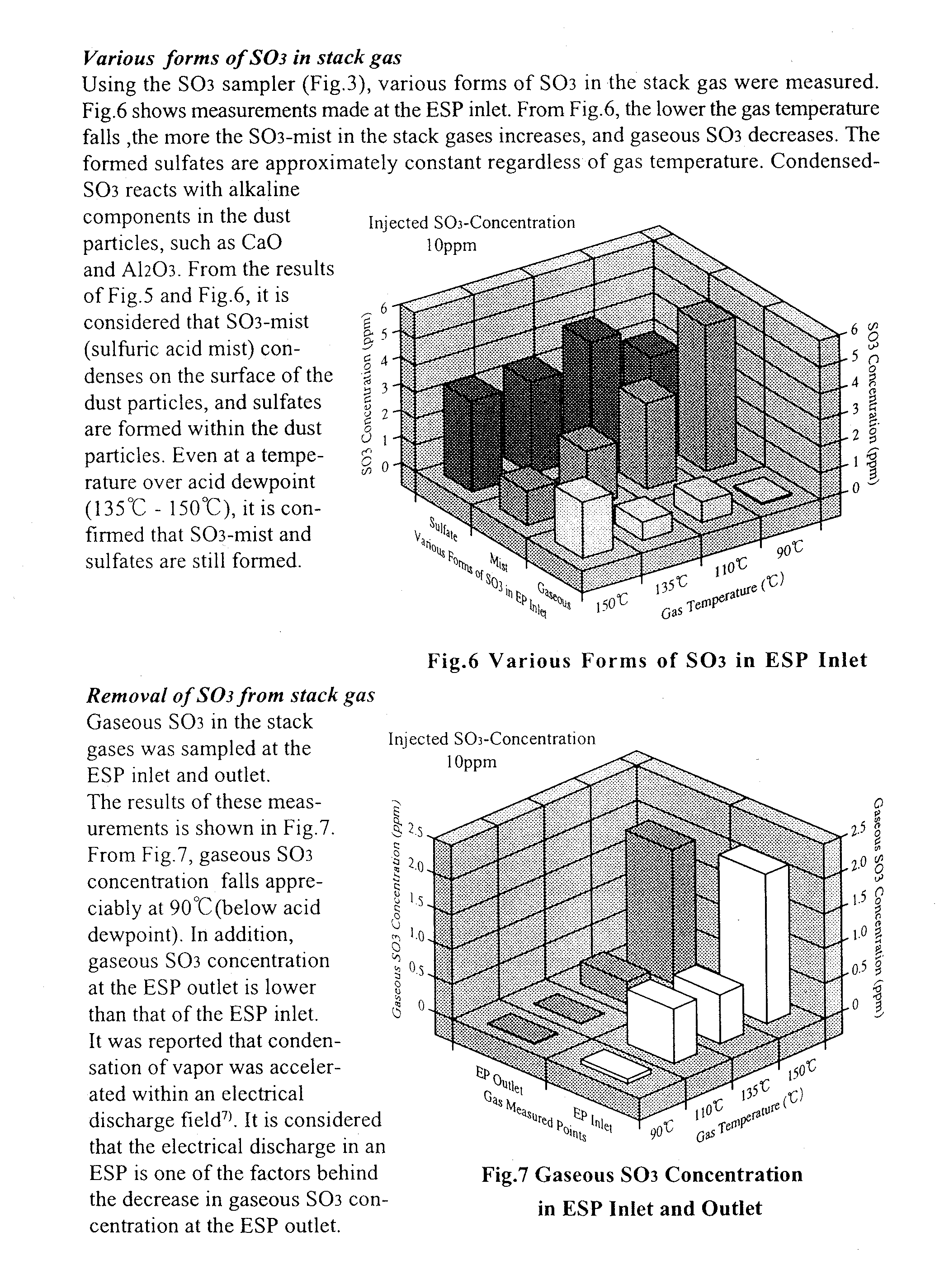
CONCLUSIONS
It became clear after the investigation on electrostatic precipitation at a temperature around acid dewpoint that the lower the gas temperature falls, the more the collecting performance of the ESP is improved. At a temperature below that of the acid dewpoint, say 90OC, the ESP can simultaneously remove both SO3 and dust particles from stack gases with high efficiency.
REFERENCES
1. Pilat, M.J. and Wilder, J.M., " Opacity of Monodisperse Sulfuric Acid
Aerosols," Atmospheric Environment, Vol.
17, No.9, pp. 1825-1835 (1983)
2. Damle, A.S., Ensor, D.S. and Sparks, L.E., " Prediction of the Opacity of Detached
Plumes Formed by
Condensation of Vapors," Atmospheric Environment, Vol. 18,
No.2, pp. 435-444 (1984)
3. Electrostatic Handbook of Japan. Institute of Electrostatics Japan, 1981
4. Verholf, F.H. and Banchero, J.T., " Predicting Dewpoints of Flue Gases,"
Chem. Eng. Progress., Vol.70, pp.
71-78 (1974)
5 . Matsumura,T., " Spectrophotometric Determination of Sulfuric Acid Aerosol in the
Atmosphere Using Barium
Chloranilate, "Ryusan to Kogyo, pp.5-9 (1980)
6. Ise, A., " Measurement of Exhaust Sulfate Emissions," Jidousya
Gijyutsu, Vol.3 1, No.5, pp.339-344 (1977)
7. Thomson, J.J. and Thomson, G.P., "Conduction of Electricity through
Gases," Vol. Cambridge Univ. Press, 310
(1928)
Back to the This Old Box page.
Back to APC Network Main Page
Last updated: April 12, 1999.
Copyright © 1997 TRK Engineering Services, Inc. All rights reserved.
For more information contact: TRK Engineering Services - 95 Clarks Farm Road - Carlisle,
MA 01741 - Telephone: 978-287-0550 - Fax: 978-287-0569 - email: trkeng@apcnetwork.com