THE SPECTRA OF THE CORONA IN AN ELECTROSTATIC PRECIPITATOR
Zhangfa Wu 1, J. K. Walters 1 and D. W. P. Thomas 2
1 Department of Chemical Engineering
2 Department of Electrical and Electronic Engineering
University of Nottingham
Nottingham NG7 2RD, UK
ABSTRACT
An optical detection system to measure the spectra of the corona in an electrostatic precipitator has been constructed. The corona light is focussed by a lens on to the input slit of a spectrograph. The output of the spectrograph is detected by a CCD unit connected to a PC for experimental run control and data processing. Preliminary results have shown that the detection system has allowed the spectra to be measured with a high signal-to-noise ratio and resolution. The ionic species present in the corona and the abundance of the individual ionic species are being identified from the measured spectra.
1. INTRODUCTION
Electrostatic precipitators have been established for many years as one of the most efficient devices of dust removal from gases. A knowledge of the ionic species present in the corona of a precipitator will provide a better understanding of corona discharge, particle charging and hence the collection performance of the precipitator.
The chemical nature of the ions can be identified by measurement of their mobility, but this is impeded by the presence of the ion wind (Gardiner and Craggs, 1977). Bastien et al. (1975) have computed, for air, the relative abundances of the various negative ion species using thermal rate coefficients. Here we present a novel method to identify ionic species by measuring the spectra of the corona. From the measured spectra, it is possible to identify the ionic species present in the corona and the abundances of the individual ionic species, and to deduce the abundance of the free electrons produced in the corona and hence of the negative ions formed outside of corona. The spectral analysis technique has been used to study the arcing phenomena in fuses (Barrow and Howe, 1991).
2. THE ELECTROSTATIC PRECIPITATOR
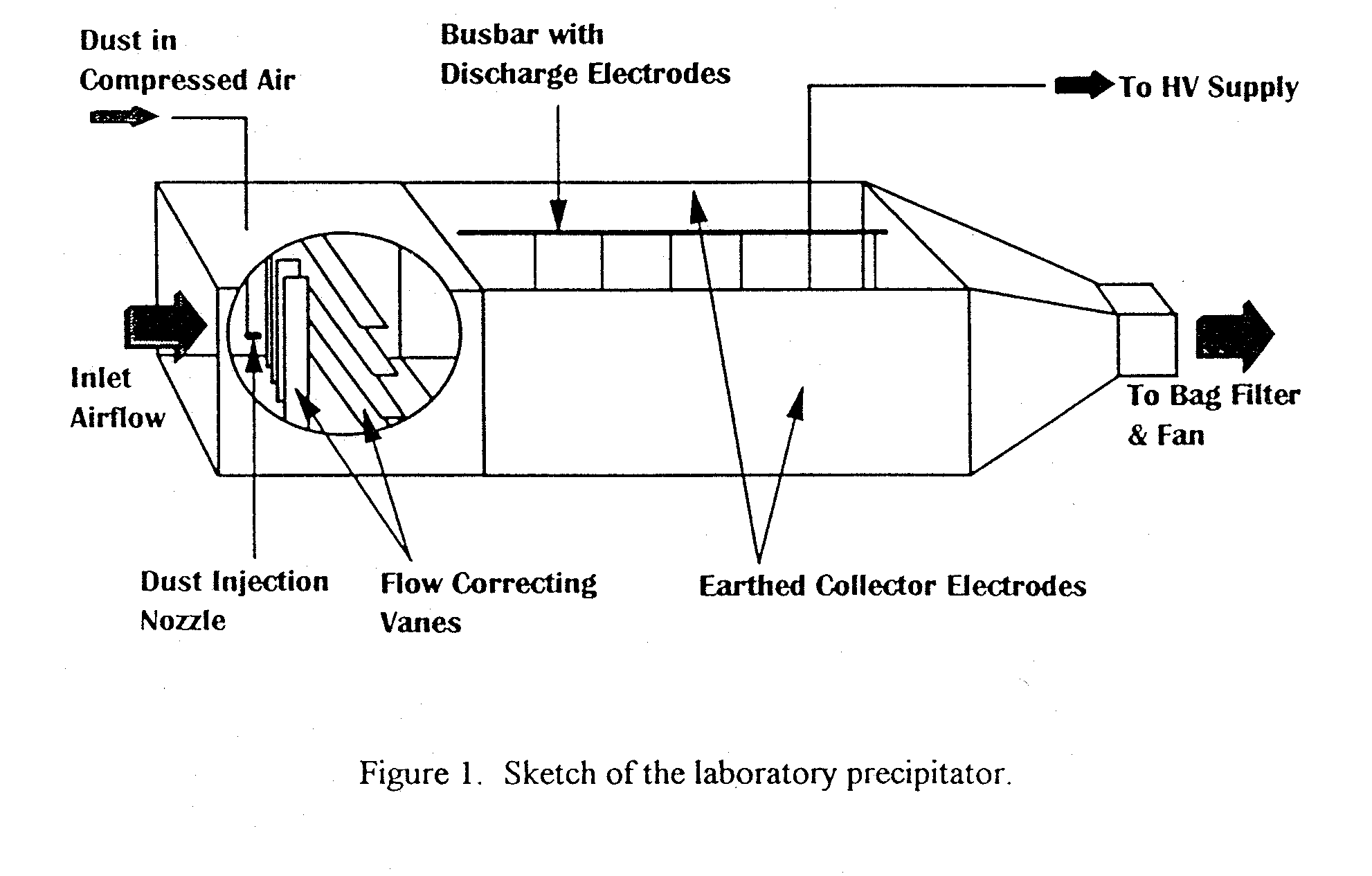
A single-stage and parallel-plate type laboratory precipitator has been constructed and used for research previously (Aldred et al, 1983; Howe et al, 1988). A sketch of the precipitator is shown in Figure 1. The unit is 4.5m long, 0.35m high and has a collector electrode spacing of 0.3m. Thus the length and width of the duct are typical of a fullscale commercial precipitator but the height is much less. The electrical section of the precipitator consists of two earthen parallel collector plates and a number of discharge electrodes which are screwed into a busbar. The busbar runs the length of the precipitator and is positioned midway between the collector plates, just below the top plane of the precipitator. This versatile design permits discharge electrodes of any shape to be inserted at any chosen spacing along the busbar. Glass plates are used as the top and bottom planes of the precipitator, which provide insulation between the discharge and collector electrodes and a visual inspection of the inside of the precipitator. At the inlet of the precipitator, flow-correcting vanes are fitted in both the horizontal and vertical planes to control the gas velocity profile in the electrical section of the precipitator. The precipitator is energised with an adjustable d.c. voltage from a transformer/rectifier unit which is capable of producing negative d.c. voltages up to 60kV at the discharge electrode.
As shown in Figure 1 the dust particles are carried by compressed air and dispersed into the gas flow at the entry to the precipitator. The dust particles are then charged by corona discharge and deposited on the collector electrodes by the electrostatic field. The treated gas flow from the outlet of the precipitator is passed through a bag filter for final cleaning before the induced-draught fan exhausts the air to the atmosphere.

3. THE CORONA DISCHARGE
The laboratory precipitator uses negative corona which is usually used for industrial electrostatic precipitators. In a small region around the discharge electrode, the field strength is sufficient high that air undergoes an electrical breakdown and hence the corona is generated. The process of corona discharge is initiated by a small amount of electrons and ions created by natural radiation. In the corona region, both the electrons and ions are accelerated by the electrostatic field, but the electrons acquire higher energy than the ions because of their much greater mean free path. Thus the electrons have sufficient energy to knock an electron from an air molecule upon collision and thereby create a positive ion and an electron. Within the corona region this process takes place in a self-sustaining avalanche which produces a dense cloud of free electrons and positive ions around the discharge electrode. The electrons stream away from the discharge electrode to the collector electrodes. As their velocity slows with the decreasing field strength at greater distances from the discharge electrode, the electrons attach to air molecules (if an electronegative gas such as oxygen is present) and form negative ions, which stream across to the collector electrodes. The positive ions produced in the corona region move rapidly to the discharge electrode and are absorbed by it. The collision of the positive ions on to the discharge electrode permits the electrode surface to liberate secondary electrons, which maintains the operation of corona discharge.
The laboratory precipitator was energised with a d.c. voltage and operated in clean air. Discharge electrodes of cylindrical shape were initially used. The laboratory was blacked out and the corona glow was then seen from both the discharge electrodes and the busbar when the precipitator was energised up to about 55kV. The glow appeared to be blue in color and was distributed in patches over the surface of the discharge electrodes and busbar. The emission of light arises from the collisions of the electrons with air molecules and from the collisions of the positive ions with the discharge electrode within the corona region. The collisions ionise or excite air molecules and the metal atoms on the surface of discharge electrode, and cause them to emit photons of characteristic wavelengths.
4. THE OPTICAL DETECTION SYSTEM
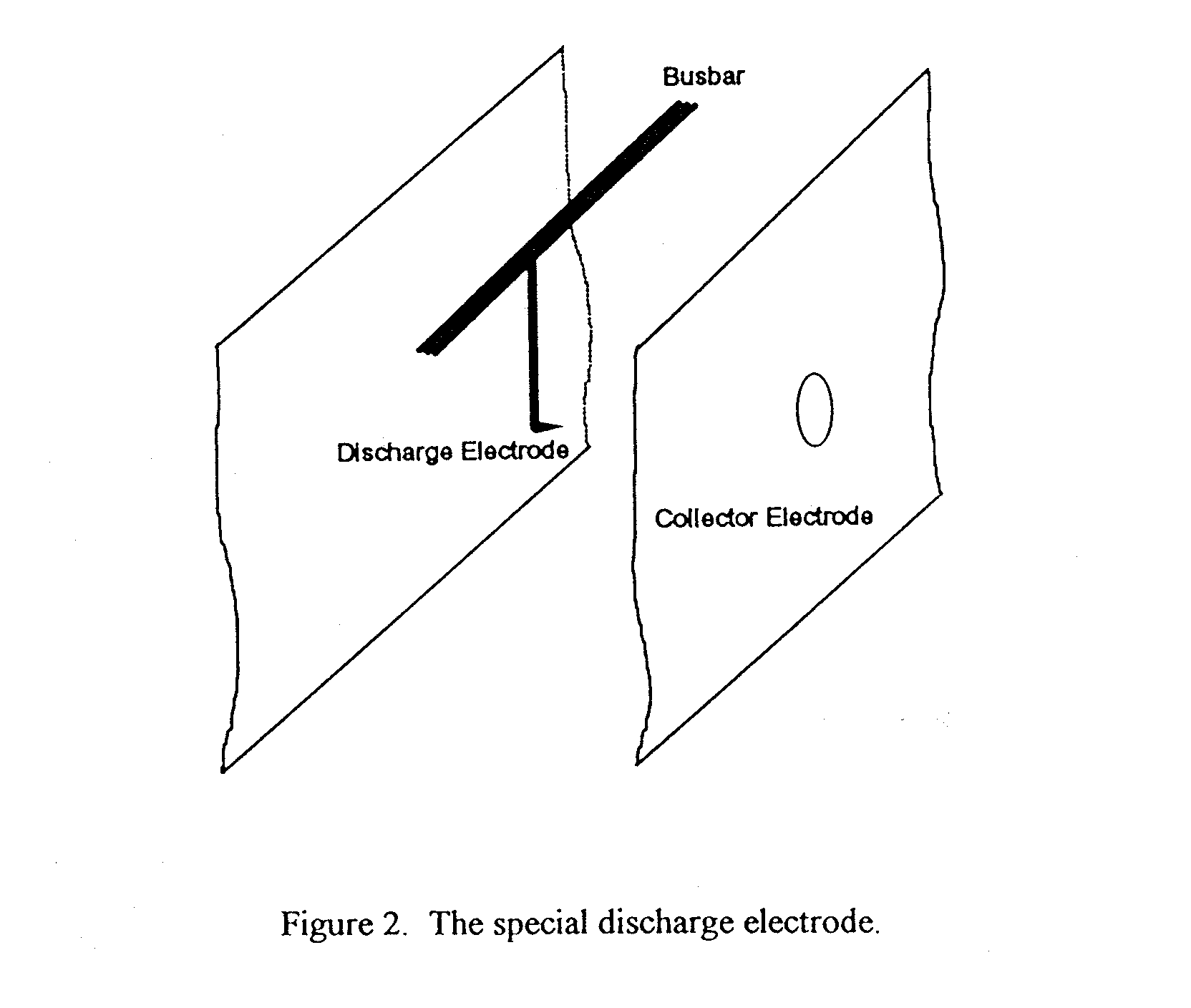
To enhance the corona emission at a known position a special discharge electrode was constructed. It is of half the length of an ordinary electrode with a point at the end facing one of the collector electrodes as shown in Figure 2. A hole was made in the collector electrode opposite the pointed electrode so that the optical system could be focussed on the enhanced corona at the point.
The optical detection system consisted initially of a lens focussing the corona light on to the end of a liquid light guide that took the light to an electrically-shielded control room. The light was then focussed on to the inlet slit of a monochromator. The output light of
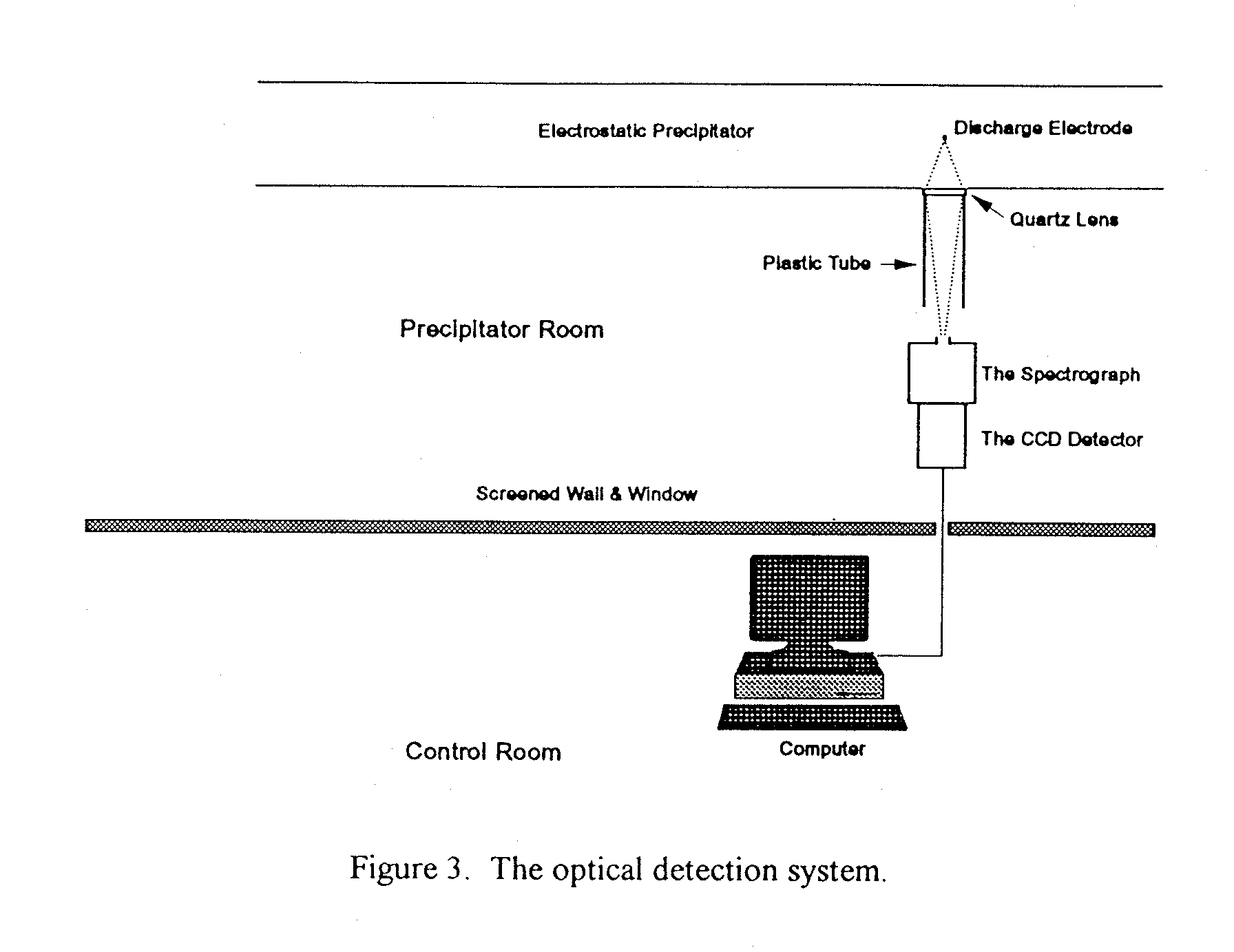
the monochromator was again focussed on to entrance slit of a photomultiplier, from which the output signal was monitored by an oscilloscope. Whilst the signal could be detected the signal4o-noise ratio was too small to obtain trustworthy results. Subsequently an InstaSpecTM IV CCD detector was used for the detection system as shown in Figure 3. The corona light was focussed by a quartz lens, which permits wavelengths down to about 200nm in the uv range to be investigated, directly on to the entrance slit of the spectrograph. Either an Oriel MultiSpec 125 Spectrograph or a custom-made monochromator could be installed as the spectrograph. In either case the range of wavelengths to be studied could be adjusted between about 200nm and 1100nm with appropriate gratings. The output of the spectrograph was then viewed by an InstaSpecTM IV CCD detector. The spectrograph and CCD detector as a whole unit were shielded in a Faraday cage close to the precipitator to dispense with the light guide and reduce the loss of light intensity. The CCD unit was connected through a shielded cable to a PC located in the Control Room, from which experimental runs were controlled and data were processed. The shielding is to prevent the detection system from static electricity and radiated electromagnetic fields arising from the high-voltage or other similar sources. The alignment and calibration of the optical system were accomplished by putting a mercury lamp at the point of the discharge electrode.
5. RESULTS AND DISCUSSION
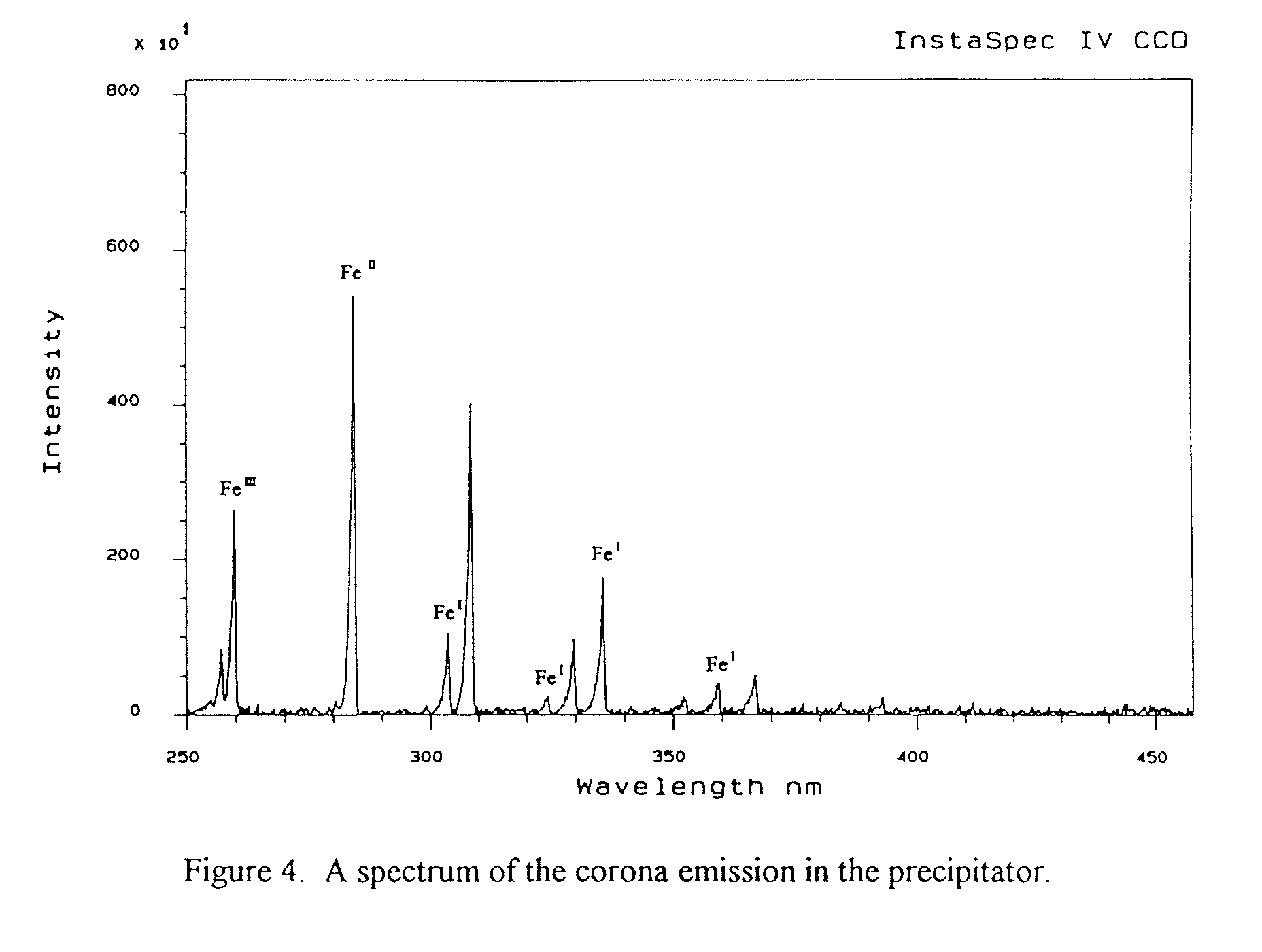
Measurements have been undertaken with the precipitator operated in ambient air and energised at various d.c. voltages from 40 to 55 kV. The preliminary results for the measured spectra in the 250 to 450 nm wavelength region are shown in Figure 4. A high signal-to-noise ratio has been clearly achieved with good resolution. However, optimization of the system is required to make the measurements more efficient. Identification of the ionic species is being made by comparison of the observed spectral lines with those found in the tables in the literature for atomic species. So far several lines for Fe ~, Fe ~ and Fe '[I have been identified, which is to be expected since the discharge electrode is made of mild steel, but other lines have still to be identified. The relative abundances of each species determined from their corresponding intensities on the spectrogram show that Fe II ions are more abundant than others. The formation of Fe
1 , Fe 11 and Fe 111 arises from the fact that the positive ions produced in the corona region collide with the mild steel discharge electrode and ionize or excite the iron atoms. It should be noted that the spectra for the molecular species, formed by the collision of electrons with air molecules, have not yet been investigated.The detection system will first be optimized and then used to measure the spectra covering a wide wavelength range between 200nm and 1100nm. From the measured spectra, both the atomic and molecular species will be identified and their relative abundance's determined. These will then be performed with different chemical composition of the gas and different materials of discharge electrode. The results will be related to the relative abundance of the free electrons produced in the corona and hence of the negative ions formed outside of corona. Finally the above investigations will be linked to measurements of the charge-to-mass ratio on different size ranges of particles and precipitator efficiency. The measurement of the charge-to-mass ratio can be accomplished by sampling particles from the precipitator, and splitting the sample into different size fractions using a cascade impactor so that the charge and mass on each fraction can be measured (11owe et al., 1988).
REFERENCES
Aldred D.C., Howe A.F. and Wright A. (1983)' Insl Phys. Conf Ser. No.66, Session VI, pp.151-154.
Barrow D.R. and Howe A.F. (1991) Proceedings of 4th Intl. Conf on Electrical Fuses and Their Applications, pp.221-225, University of Nottingham, 23-25 September.
Bastien F., Haug R. and Lecuiller M. (1975) J Chim. Phys., 72, pp.105-112.
Gardiner P.S. and Craggs J.D. (1977) J Phys. D: Appl Phys., 10, pp.1003-1009. Howe A.F., Houlegreave J.A. and Walters J.K. (1988) Filtration Society Meeting,
London, 24 May.